From sample acquisition to Molecular Tumor Board
Transforming Precision Oncology Practice
Hands-on training for key opinion leaders in molecular diagnostics and clinical application in oncology
Preceptorship Pilot Program 2024
Who we are
Vessel's job is to educate patients and enable clinicians to implement state-of-the-art precision oncology approaches throughout the entire patient journey, driving the adoption of precision medicine by hospitals, payers and providers.
Clinicians need to accept and overcome the fact that precision oncology asks for polymath-like proficiency across multiple knowledge domains. If you want to employ these methods today and increase patient impact, you need to bridge the gap between the technology and clinical application - and Vessel is this connective tissue across all knowledge domains and stakeholders.
Our Promise
We achieve this through a unique blend of on-site training, immediately applicable digital content, and a dedicated community.
Program Overview
We are launching our first-ever on-site training program at the Division of Oncology, Medical University of Graz. The program consists of 8 modules that reflect the end-to-end clinical workflow for routine cancer diagnostics in the advanced cancer setting.
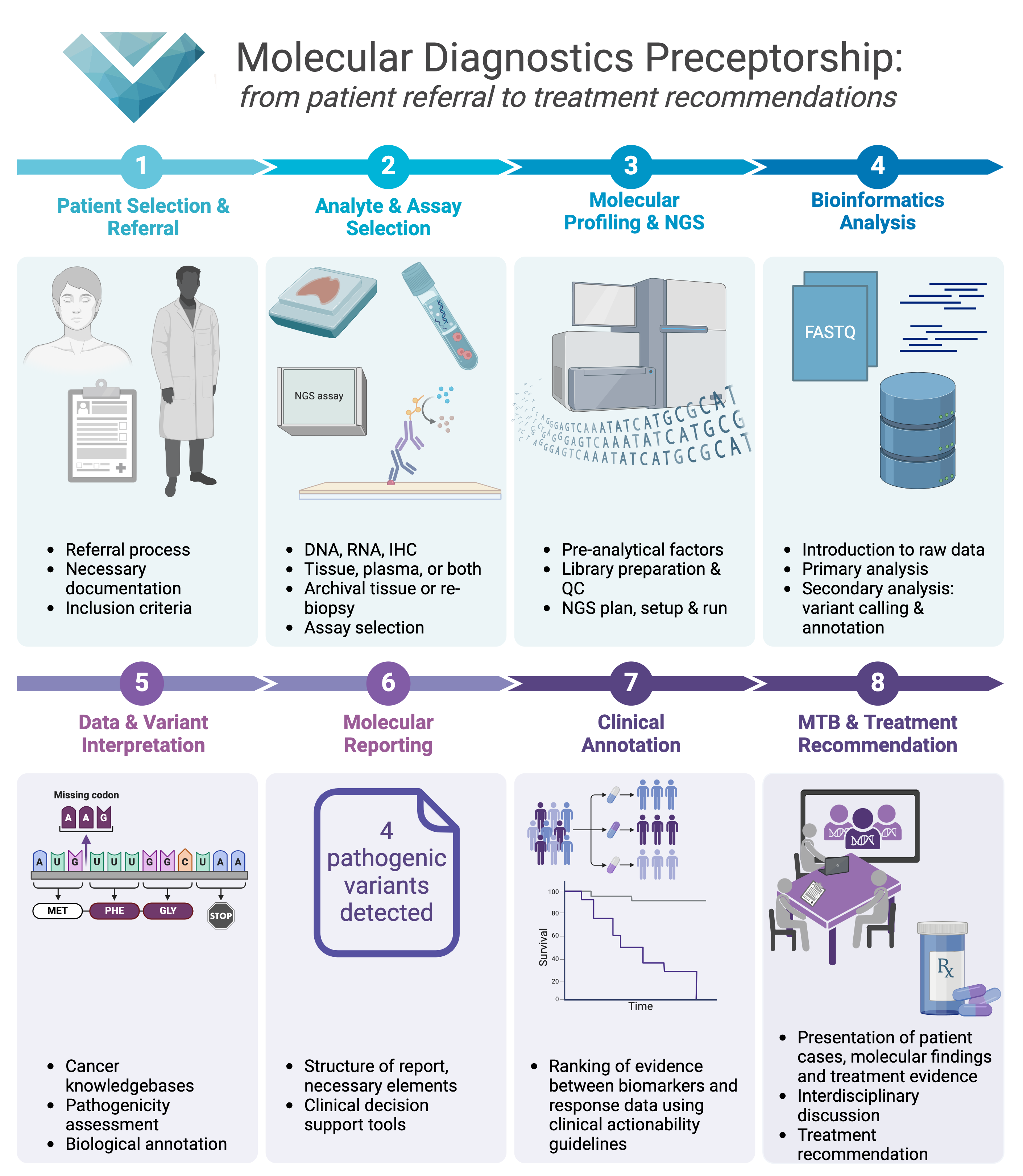
Modules 1-4 represent the steps from patient referral, to the next-generation sequencing (NGS) wet lab, to NGS secondary analysis. Modules 5-8 represent the steps from NGS tertiary analysis, to clinical report generation, to the molecular tumor board. Below is a more detailed description of the types of questions that training in each individual module will answer:
Module 1 - Patient Selection and Referral
- Which patients should receive further diagnostic testing?
- What is the process of referring patients to the molecular tumor board (MTB)?
- What are the patient inclusion criteria that the MTB employs to select patients for further testing?
- What type of patient information and history is necessary to process these requests?
Module 2 - Analyte & Assay Selection
- How do you select the appropriate analyte and assay for a patient-specific diagnostic workup?
- How do you select the right tissue blocks and when do you perform re-biopsy?
- Should you analyze DNA, RNA, or both?
- Which NGS assay should be ordered?
- Should you also perform IHC testing of common biomarkers?
- When should you use liquid biopsy/ctDNA testing approaches?
Module 3 - Molecular Profiling and NGS
- What aspects of sample collection and processing are important for performing NGS assays?
- What types of library preparation are out there, how are these steps performed and how do you perform QC of this step prior to sequencing?
- How do you plan, set up and carry out an NGS run?
Module 4 - Bioinformatics Analysis
- What does NGS raw data look like and how do you perform QC on these data?
- What is the difference between primary, secondary and tertiary NGS analysis?
- How do you go from raw base calls from the sequencer to variant and copy number calling?
- What kind of bioinformatics workflows and know-how are necessary to carry out clinical testing?
Module 5 - Data and Variant Interpretation
- How do you put a detected variant into clinical context and determine its pathogenicity?
- How do you annotate the alterations you detect with biological and clinical information?
Module 6 - Molecular Reporting
- What should a final molecular cancer diagnostics report look like?
- Which elements are essential and which are of additional value for the clinician?
- What type of automated tools are out there that help streamline the variant interpretation and clinical annotation steps?
- How do these so-called clinical decision support tools work?
Module 7 - Clinical Annotation
- How do you annotate and rank the detected alterations in a molecular report based on clinical evidence?
- Which alterations are actionable and how do you match these to targeted treatments?
- What are clinical actionability guidelines and how are they used in the routine setting?
- What types of resources can you query in order to prioritize clinical evidence of biomarkers?
Module 8 - MTB and Treatment Recommendation
- What is the purpose, structure and function of the interdisciplinary molecular tumor board (MTB)?
- How frequently does it meet, what types of cases does it discuss, what are the roles of each member, and how does it determine individualized evidence-based treatment recommendations for each patient based on biomarker data?
- What is the necessary infrastructure for an MTB?
- What current challenges does the MTB face in implementing precision oncology?
Interested? You can pre-register today and our team will follow up with you on the details.
Important things to know
Location
The Vessel preceptorship "From sample acquisition to Molecular Tumor Board" will take place in Graz, Austria at the Medical University of Graz and is hosted by Prof. Philipp Jost and the Division of Oncology, Department of Internal Medicine.

Schedule
Our program will be offered year round. Once you have completed the pre-registration, we will follow up with you personally with a schedule that you can choose from.
Cost
Our current program is sponsored by our industry partners. Obtaining a sponsored seat is subject to our application process. You can express your interest and reserve your spot today:
If you are selected, our team will take care of travel and accommodation planning.

